OSSGROW® Bone Cell Therapy can preserve your patient's hip

As per USFDA and EMA, Avascular Necrosis has no approved standard of care. Also, there is no approved drug or biosimilar in the global market to treat this condition.
There is an urgent need to develop a biological cure for AVN.

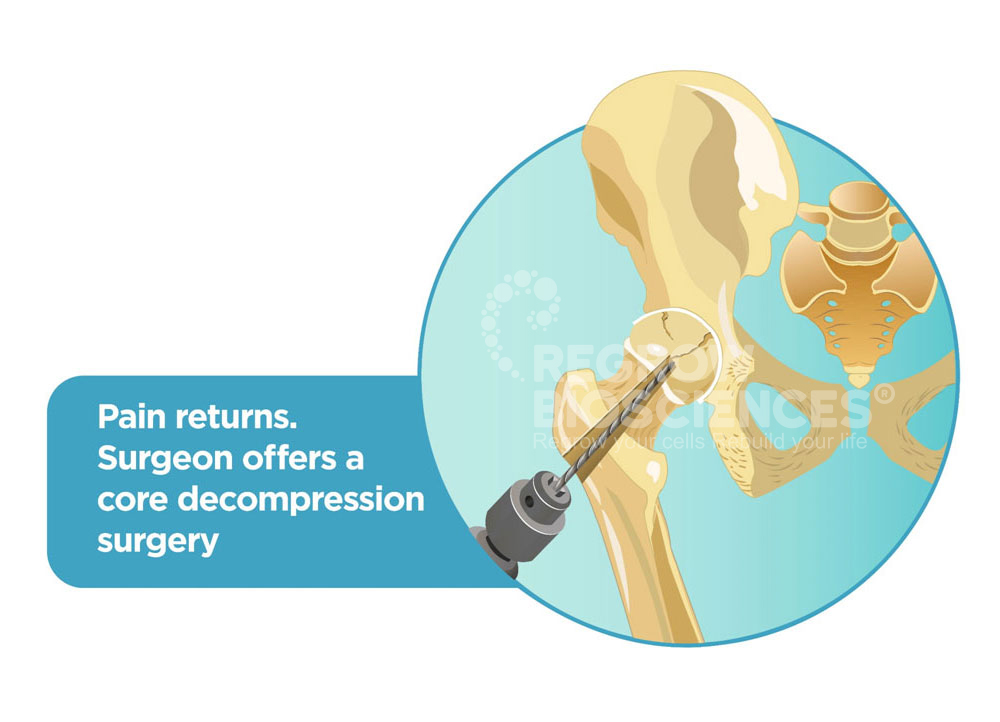
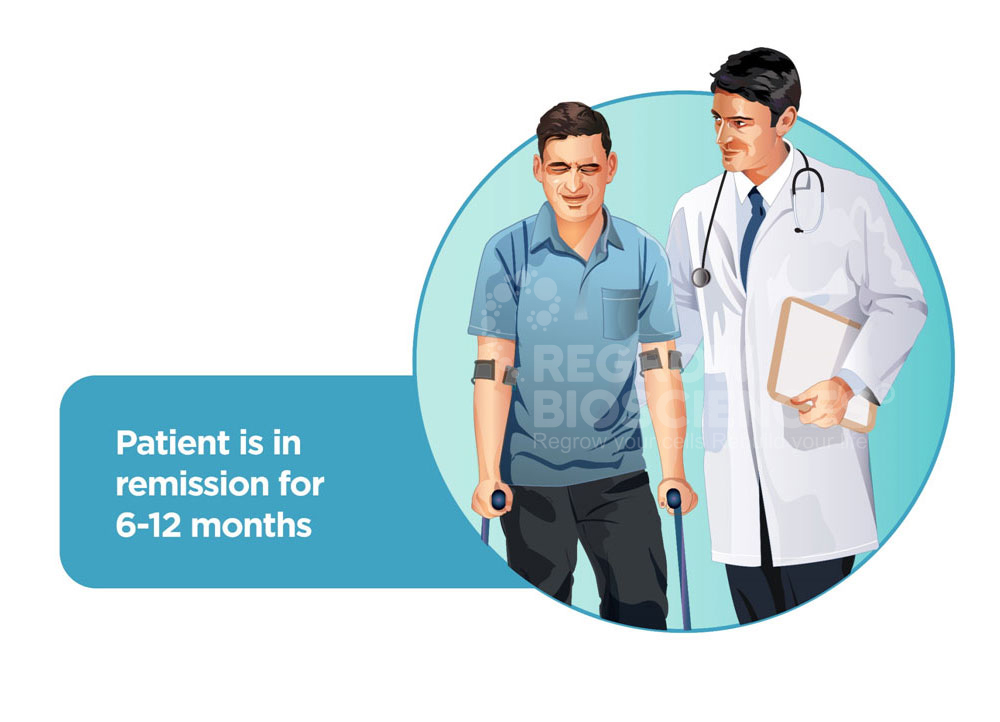
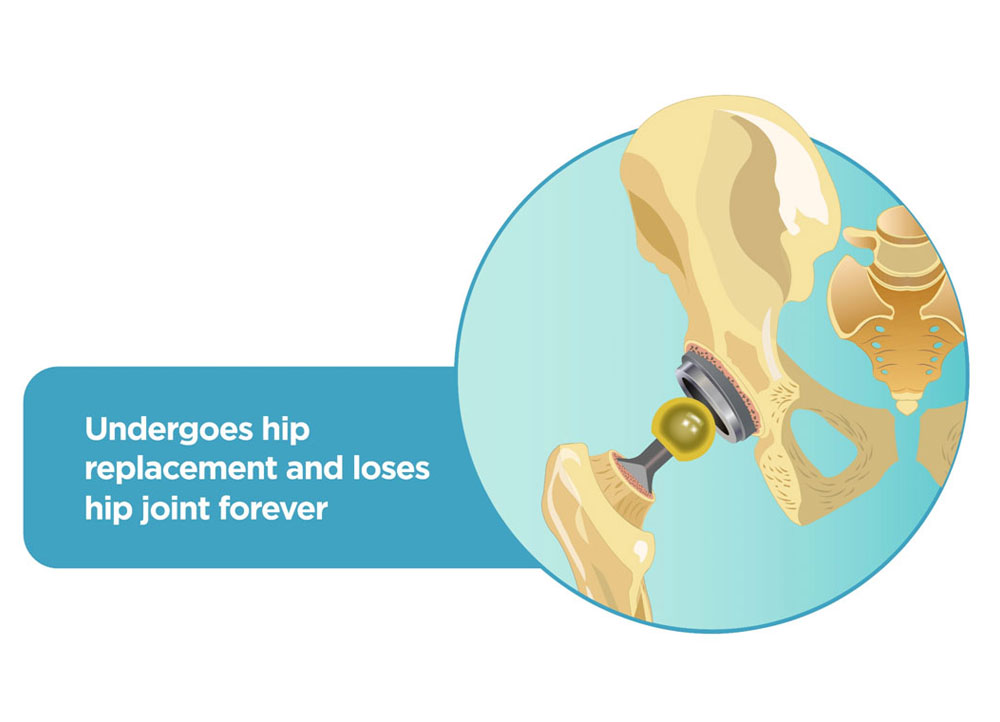
Majority of patients with AVN undergo multiple surgeries. Starting off with a simple core decompression which gives temporary relief for only 2-3 years, patients have to undergo a hip replacement surgery in their mid 30’s, risking their mobility and career.
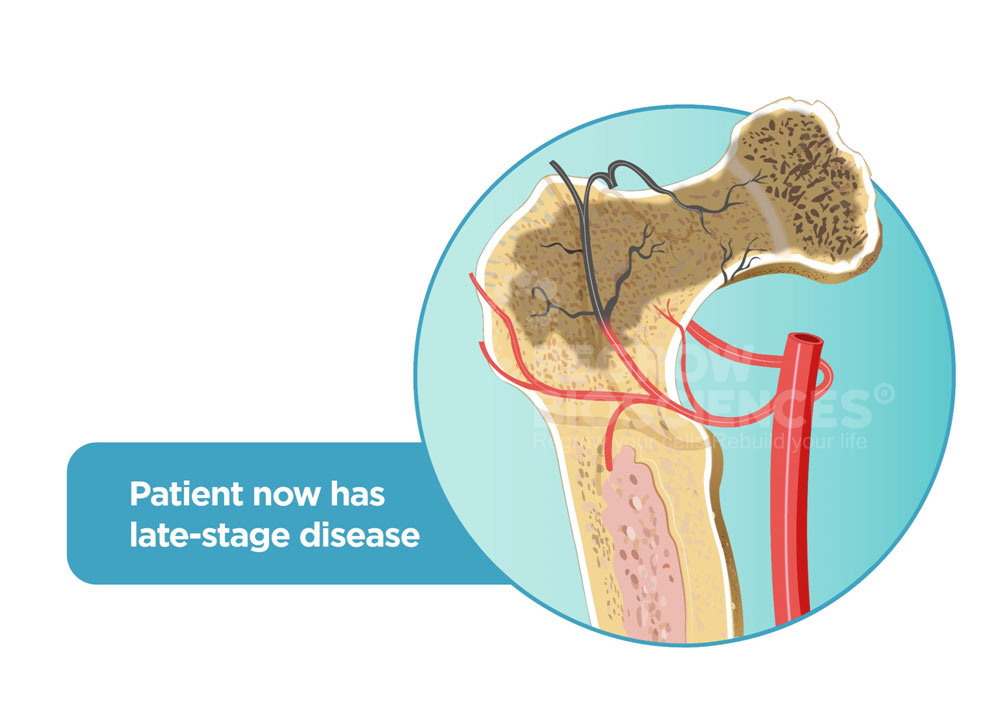
Thereafter, >30% patients undergo revision hip surgery in 5-7 years resulting in mental & financial collapse.




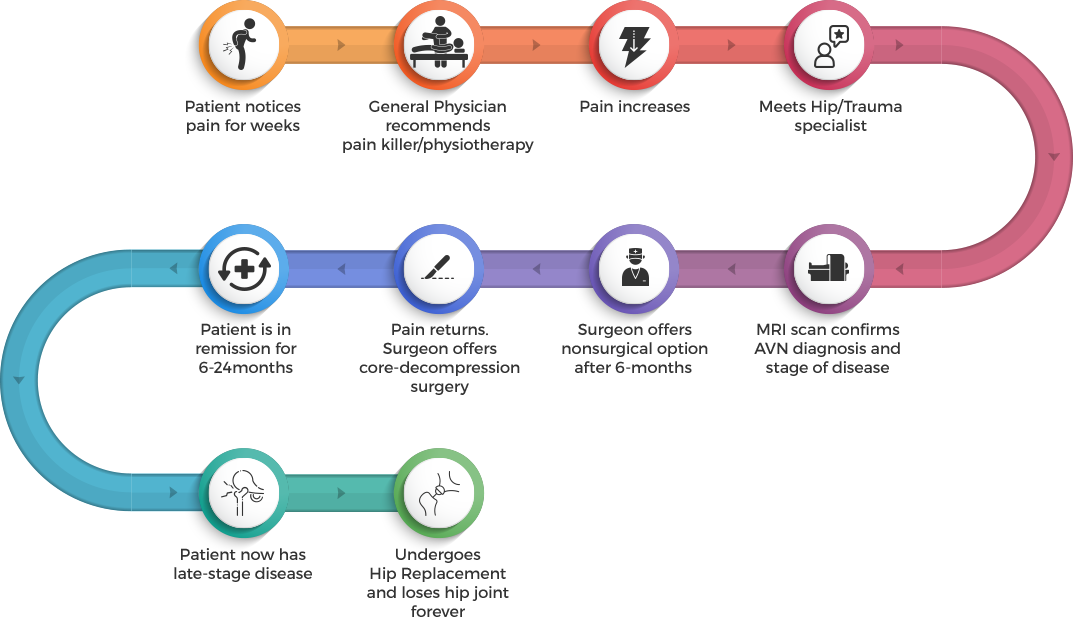







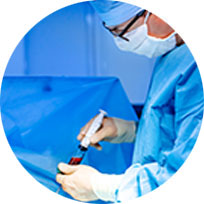
Bone Marrow harvested from the iliac crest. The Bone Marrow is a rich source of osteoprogenitor cells such as mesenchymal stem cells.

Osteoblasts cell culture in a GMP cell processing facility takes 3-4 weeks. The final product consisting of a highly characterized homogeneous cell population is transported to the hospital/treating physician.
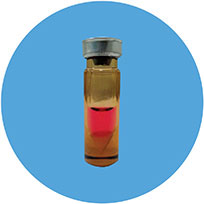
The cultured autologous osteoblasts (Ossgrow®) are injected into the patient’s hip through a minimally invasive step involving core decompression.
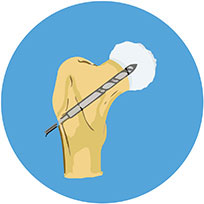
The injected osteoblasts convert into an organic mineralized component of the bone called osteoid leading to mature bone formation.
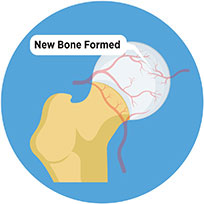
Through bone cell therapy, the structure and function of the hip joint is regenerated and restored preserving them.

48 Million cells per hip joint

Confirming to Microbial Sterility and Genetic Stability

Homogeneous population of osteoblast cells

Bone Alkaline Phosphatase+ marker expression.

Positive expression of Osterix and Runx2 Genes.

One-time implantation only
ICH-GCP compliant phase III clinical trials successfully completed.
No product-related adverse events or serious adverse events were reported during the clinical trials.
Primary efficacy endpoints were achieved
Product is commercially available and marketed in India with possibility of registration in other countries of interest.
Read the Clinical Trial Results & Phase 3 CT Prospective Study here
Administration of OSSGROW® in patients affected with AVN demonstrated its safety and efficacy in actual clinical practice.
No product safety related issues
No patient safety related issues
PMS of 50 patients was successful with no safety related observations (across 14 hospitals)
No abnormal growth or overgrowth of bone tissue was observed in the long-term study.
Mediclaim Cashless and Mediclaim reimbursement

On GEM portal for government hospital procurement

in 300+ Hospitals across India.